Introduction:
Patients with hematological malignancies are at an increased risk of morbidity and mortality after COVID-19 infection.Especially patients treated with chimeric antigen receptor T-cell (CAR-T) therapy for B-cell malignancies have shown poor outcomes after COVID-19, resulting in reported COVID-19 attributable mortality rates between 41%-50%.Outcomes after COVID-19 infection in patients treated with CD20xCD3 bispecific antibodies have not been reported. We investigated the impact and clinical course of COVID-19 in 130 Danish patients with B-NHL who have received bispecific antibodies.
Methods:
Using clinical data from electronic health charts, the national Danish database of microbiology (MiBA), and the national drug- and vaccination registry (SharedMedicineCard), we collected data on date of positive COVID-19 test, quantitative PCR-determined COVID-19 variant, symptoms, reactivations, resolution of disease (either clinical, confirmed by negative PCR test or death), vaccination status and treatment. Disease duration was defined as the time from diagnosis of COVID-19 to clinical resolution.
Results:
A total of 130 patients who received bispecific CD20xCD3 antibodies (glofitamab or epcoritamab) as part of clinical trials in Denmark were assessed. Of the 130 patients, 109 were alive after March 8, 2020. We identified 43 patients infected with COVID-19, 34 of whom were during CD20xCD3 specific treatment or after end-of-treatment (Table 1). A total of 26 patients (79%) were in complete remission at the time of COVID-19 diagnosis, 5 patients (15%) had progressive disease, 2 patients (6%) had stable disease and 1 patient had no data on lymphoma status at COVID-19 diagnosis. With regards to COVID-19 symptom burden, 4 patients (12%) were asymptomatic during infection. The most common symptoms included fever (n=15), cough (n=12), and dyspnea (n=6). There were 16 patients (48%) admitted to the hospital for the treatment of COVID-19. Of these, 2 patients required oxygen support. Twelve patients (35%) received no specific treatment for COVID-19, while the most commonly prescribed treatments were sotrovimab in 13 patients (42%) and remdesivir in 9 patients (29%).
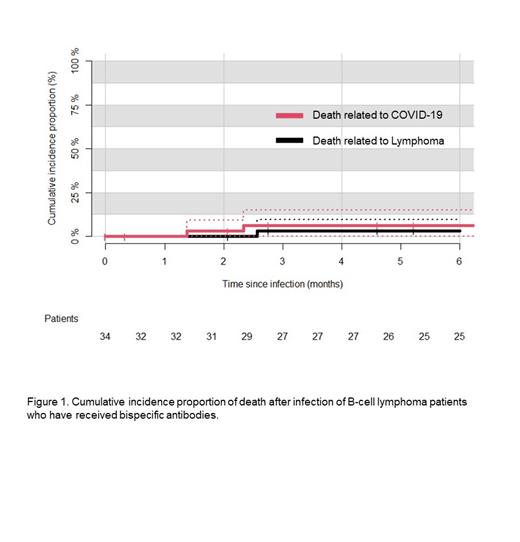
At six months after COVID-19 infection, 3 patients had died, two before recovering from COVID-19. The two cases, where COVID-19 presumably was a cause of death, had a combination of multiple bacterial infections and progression of malignant disease. The 6-month cumulative incidence of death related to COVID-19 was 6.4% (95% CI 0% to 14.9%, Figure 1), while the cumulative incidence of death overall was 9.6% (95%CI 0% to 19.9 %). Of the 25 patients who were alive and had data available at 6 months follow-up after COVID-19 infection, 20 were in complete remission, while the rest (n=5) had refractory/relapsed lymphoma.
Of the patients with COVID-19 during CD20xCD3 specific treatment or after end-of-treatment, 13 out of 34 had at least one reactivation. The mean number of COVID-19 reactivations was 1.2 (95%CI 0.44 to 2.03), ranging from 0 up to 9. An additional patient had a reactivation 20 days after first infection and 4 days after first treatment with bispecific antibodies. Only 9% (1 out of 11) of the patients who tested positive for SARS-CoV-2 before bispecific antibody treatment had a reactivation compared to 38% of those testing positive after or during bispecific antibody treatment. In an adjusted multivariable regression for age and sex only hospitalization during the first COVID-19 infection was significantly associated to reactivations (OR: 18.9, 95%CI 2.84-238, p=0.007).
Conclusions:
To our knowledge, this study presents the first data on COVID-19 incidence and severity in patients with relapsed/refractory lymphoma who have received CD3xCD20 bispecific antibodies. The COVID-19 attributable mortality after bispecific CD20xCD3 antibody therapy, when compared to CAR-T cell therapy, was much lower and comparable to the mortality reported in other cohorts of less heavily treated hematological malignancies infected with omicron variants. However, these patients present with considerable number of COVID-19 reactivations. In conclusion, it seems relatively safe to prescribe bispecific CD20xCD3 antibodies for lymphoma for patients who have been vaccinated for COVID-19 with the currently circulating virus variants.
Disclosures
Clausen:AbbVie, Janssen, Gilead, Astra Sencea, Genmab, Roche, Incyte:: Consultancy. Niemann:Carsten Niemann has received research funding and/or consultancy fees from AstraZeneca, Janssen, AbbVie, Beigene, Genmab, CSL Behring, Octapharma, Takeda, and Novo Nordisk Foundation.: Consultancy, Research Funding. Grønbæk:Kirsten Grønbæk received research support from Janssen and is on the advisory board of Nanexa and GSK.: Consultancy, Research Funding. Hutchings:Martin Hutchings has a Consulting or Advisory Role at Takeda, Roche and Genmab and has received Research Funding from Celgene, Genmab, Roche, Takeda and Novartis.: Consultancy; AbbVie, AstraZeneca, Celgene, Genmab, Janssen, Merck, F. Hoffmann-La Roche Ltd, Takeda: Consultancy; AbbVie, AstraZeneca, Bristol Myers-Squibb, Celgene, Genentech, Genmab, Incyte, Janssen, Merck, Novartis, F. Hoffmann-La Roche Ltd, Takeda: Research Funding; AbbVie, AstraZeneca, Celgene, Genmab, Janssen, Merck, F. Hoffmann-La Roche Ltd, Takeda: Honoraria; AbbVie, AstraZeneca, Celgene, Genmab, Janssen, Merck, F. Hoffmann-La Roche Ltd, Takeda: Membership on an entity's Board of Directors or advisory committees.